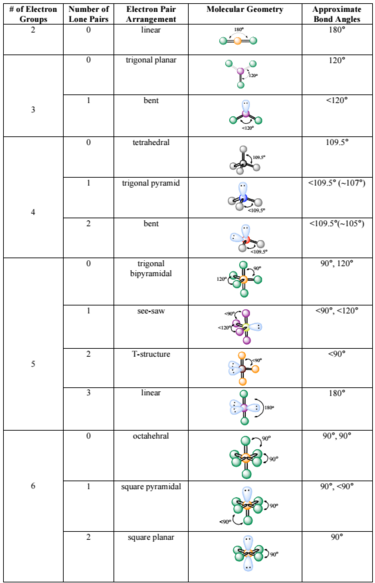
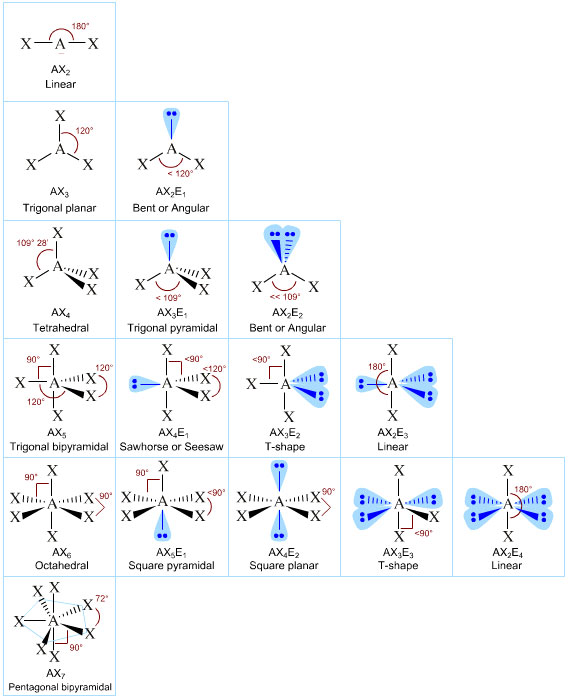
Electron Pair Geometry Chart
Electron Pair Geometry Chart. The electron-pair geometry provides a guide to the bond angles of between a terminal-central-terminal atom in a compound. The definitions of an electron pair is electrons that are in pairs or multiple bonds, lone pairs and sometimes even just one single electron that is.

Here, we have a diagram giving us the VSEPR chart: According to the chart, we get a bent molecular structure for NOCl.
The number of orbitals is always conserved.
If we look at the molecule, the O=N and the Cl-N bonds stretch to form a linear structure. If a molecule contains atoms only bonded to the central atom and no lone pairs present, the molecular geometry and the electron-pair geometry are the same. The orbitaIs comprising the numerous developing and nonbonding sets in the valence covering will prolong out.
Rating: 100% based on 788 ratings. 5 user reviews.
Veronica Cain
Thank you for reading this blog. If you have any query or suggestion please free leave a comment below.







0 Response to "Electron Pair Geometry Chart"
Post a Comment