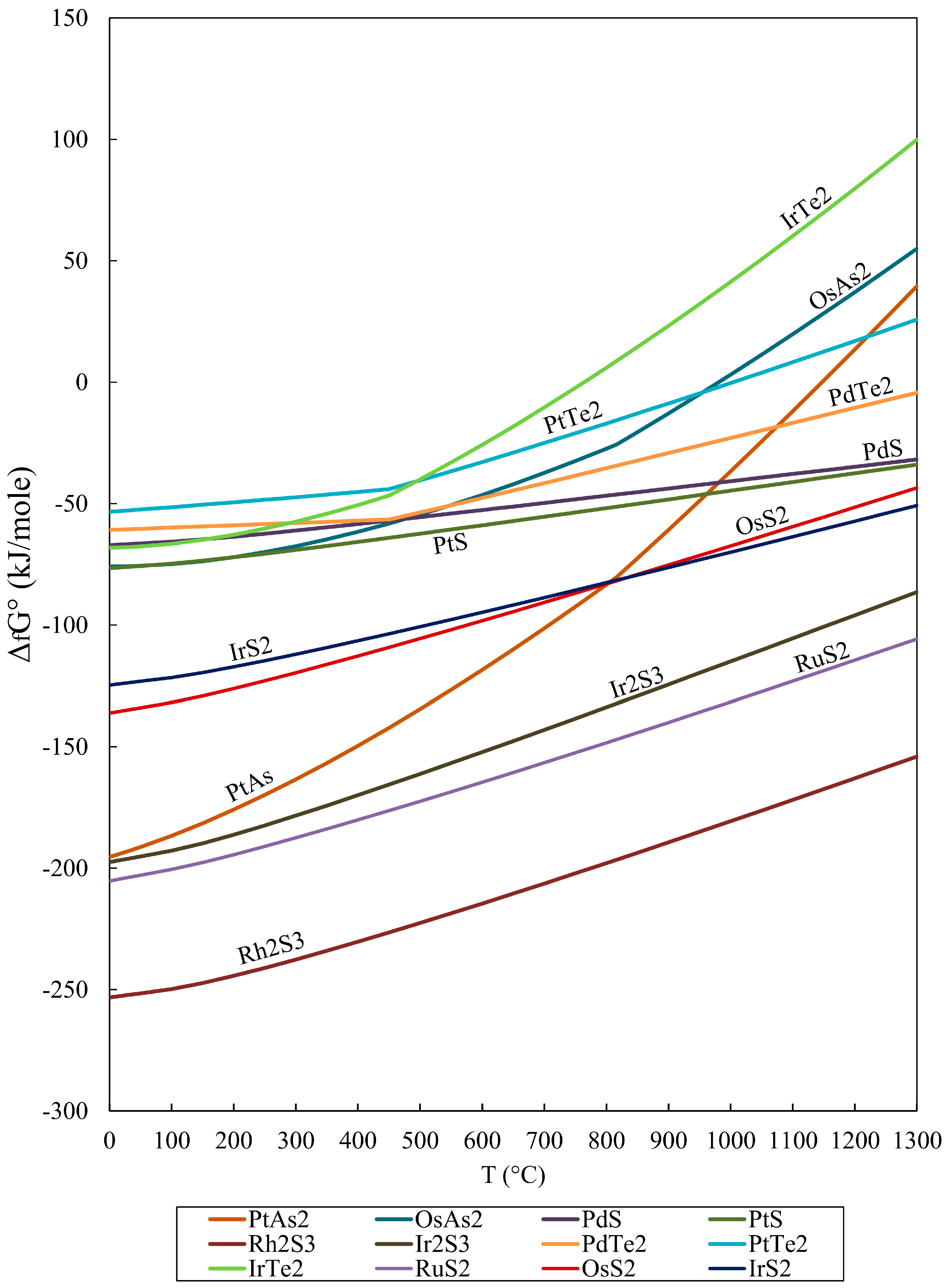
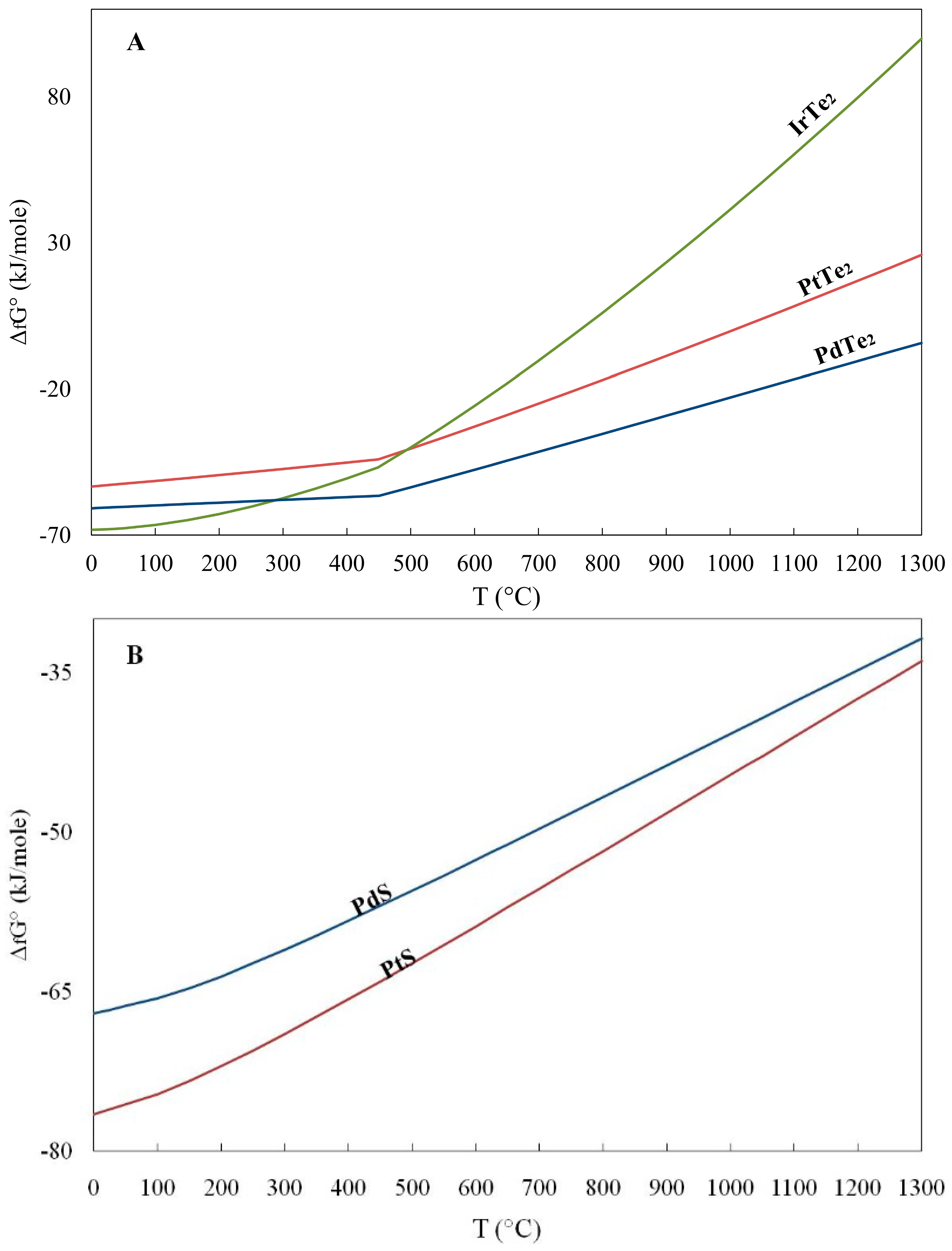
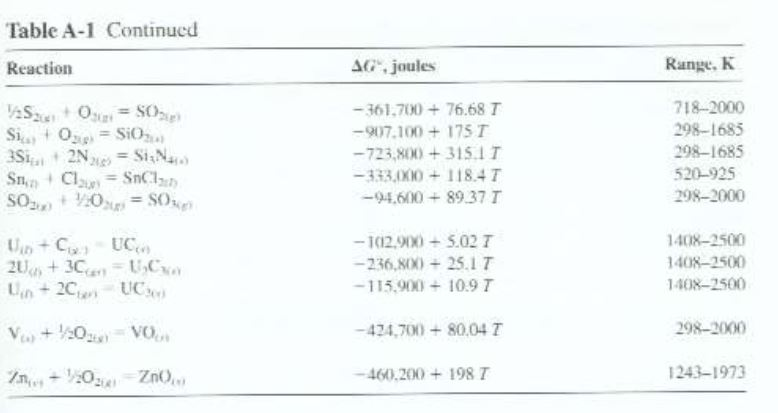
Gibbs Free Energy Chart
Gibbs Free Energy Chart. MN is the section of the surface of dissipated energy. This is a spontaneous, exergonic reaction.

Gibbs free energy is denoted by the symbol 'G'.
Joules or Kilojoules are the units of energy.
PLAY. if ΔH is negative and ΔS is positive, ΔG is. always negative/spontaneous. ifΔH is positive and ΔS is negative, ΔG is. always positive, nonspontaneous. when the situation is indeterminate, a low temperature favors the _____ factor and a high temperature favors the _____ factor. substrates and products can change the free energy release of a reaction. The exact form of the GibbsFreeEnergy function depends on the substance and independent variable (s) selected. The Gibbs free energy of a system at any moment in time is defined as the enthalpy of the system minus the product of the temperature times the entropy of the system.
Rating: 100% based on 788 ratings. 5 user reviews.
Veronica Cain
Thank you for reading this blog. If you have any query or suggestion please free leave a comment below.






0 Response to "Gibbs Free Energy Chart"
Post a Comment